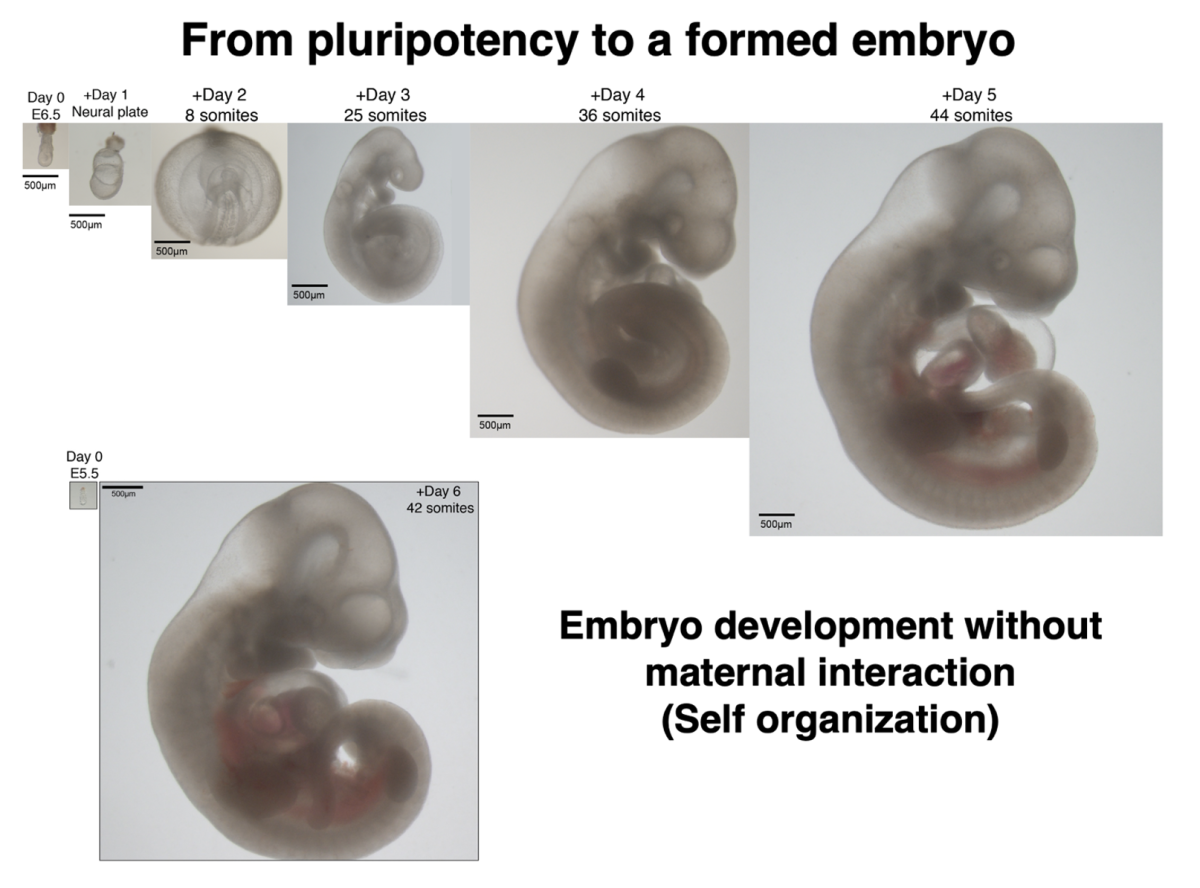
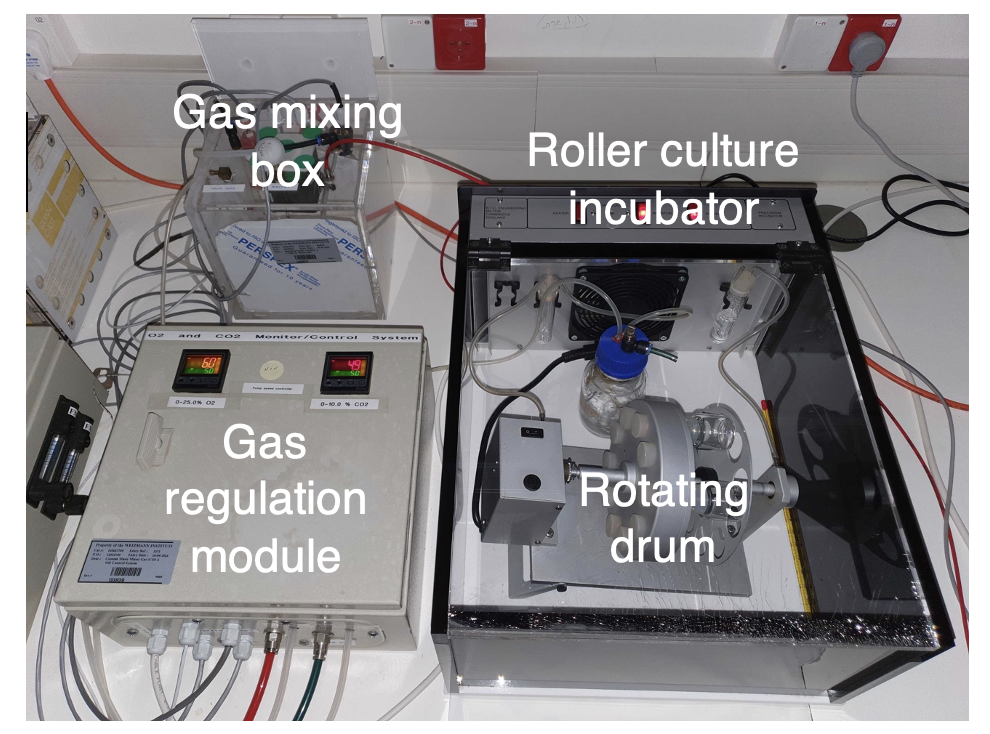
Studying mammalian development is crucial for understanding the basis of developmental defects and for devising protocols for inducedpluripotent stem cell (iPSC) in vitro differentiation which can be used for cell transplantation. The inaccessibility of the advanced natural mammalian embryo due to its intimate association with the maternal uterus, that constitutes a “black box”, has been a major constraint for investigating mammalian development at high resolution from all mammalian species. Our group has recently established a robust electronically controlled ex utero whole embryo culture platforms that allow capturing normal mouse embryogenesis from pre-gastrulation (E5.5) until advanced organogenesis (E11) ex utero (Aguilera-Castrejon et al. Nature 2021).
Our work established for the first time in a mammalian species, i.e. in mice, that the processes of gastrulation and organogenesis can be jointly and continuously recapitulated adequately in the petri dish. Importantly, these systems allow introducing genetic perturbations at early stages of development ex utero and following their effects after culture until advanced stages, rendering them highly relevant for mouse embryology and stem cell research. We view the ability to continuously capture and perturb mouse gastrulation and organogenesis ex utero as a newly revived area of research that necessitates further extensive technology development in order to harness the full potential of this biotechnology and extending it to other mammalian model organisms that better resemble human embryo development (e.g. Rabbits and monkeys).