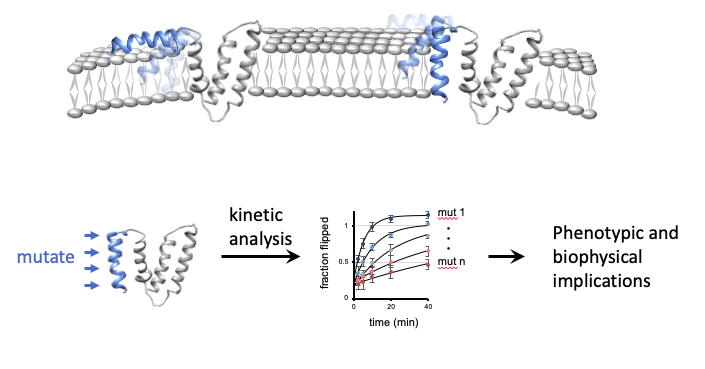
The basic structural unit of membrane proteins is the hydrophobic transmembrane helix. Until recently, it was believed that the membrane greatly constrains transmembrane helices, preventing them from flipping across the membrane or changing their transmembrane topology. Recently however, surprisingly dynamic helices have been observed, especially for unfolded membrane proteins. We have developed a method to kinetically follow helix flipping events. Our studies raised the hypothesis that helix flipping events are widespread, particularly before the protein stably folds, and that they may facilitate multiple biological roles. Essential roles for helix flipping have previously been identified in the mechanisms of apoptosis, phage lysis, and antimicrobial peptides, but we suspect that many novel functions await discovery. We seek to illuminate this fundamental overlooked conformational freedom of membrane proteins, by systematically investigating the biophysical basis of helix flipping and the roles these dynamics play in specific biological mechanisms. We combine low and high-throughput protein engineering to reveal how mutations affect helix flipping kinetics, and how these changes in flipping eventually alter the protein’s function and cell fitness.