Molecular oxygen is kinetically quite stable towards reaction at room temperature because of its triplet ground state and strong oxygen-oxygen bond. On the other hand the thermodynamic tendency for the reaction of O2 is combustion, that is to form carbon dioxide (CO2) and water (H2O). Thus, hydrocarbons typically react with O2 via a complex free-radical pathway termed autooxidation. These reactions are usually not selective and often have little synthetic utility. In order to overcome this limitation, in our group we are developing catalysts that catalyze reactions by new reaction pathways. Catalysis is used both to lower the activation energy of the reaction and to change the chemoselectivity of the reaction.
We are studying three different approaches to Oxygen Activation and Aerobic Oxidation
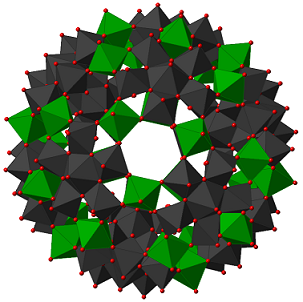
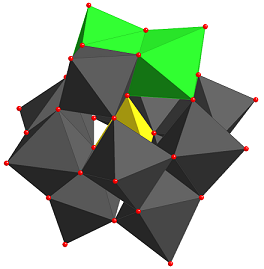
(a) Electron Transfer-Oxygen Transfer Pathways Catalyzed by Polyoxometalates for Hydrocarbon Functionalization.
The general methodology for aerobic oxidation reactions by such mechanisms involves activation of a substrate, typically a hydrocarbon, by electron transfer. In earlier work described in the 1990’s this electron transfer led to a dehydrogenated product while concomitantly the reduced catalyst was reoxidized by O2. More recently we were able to show that the electron transfer oxidation of the hydrocarbon substrate can be coupled to an oxygen transfer from the polyoxometalate catalyst by a mechanism we now term an electron transfer-oxygen transfer (ET-OT) mechanism. This is a unique mechanism in liquid phase oxidation Some salient features of these reactions are that (a) electron transfer or reduction of the catalyst is needed for subsequent oxygen transfer and (b) the catalyst can be recycled by O2. Such concepts were extended to novel reactions for example the oxidation of primary and vicinal alcohols by C-C bond cleavage rather than by the very prevalent C-H bond activation useful for deconstruction of biomass to useful intermediates
(b) Reductive Activation of Molecular Oxygen
Collectively, monooxygenases have diverse structures and active site configurations but it can be generalized that molecular oxygen activation takes place via the bonding of O2 to a Fe(II) or Cu(I) center, followed by lysis of the O-O bond under reducing conditions to yield a highly active oxygen donating species for hydrocarbon oxygenation. In our research we use both iron-containing and copper-containing polyoxometalates to electrochemical activate oxygen under reducing conditions to oxidize alkanes, alkenes and other compounds at room temperature, including components of natural gas such as methane and ethane.