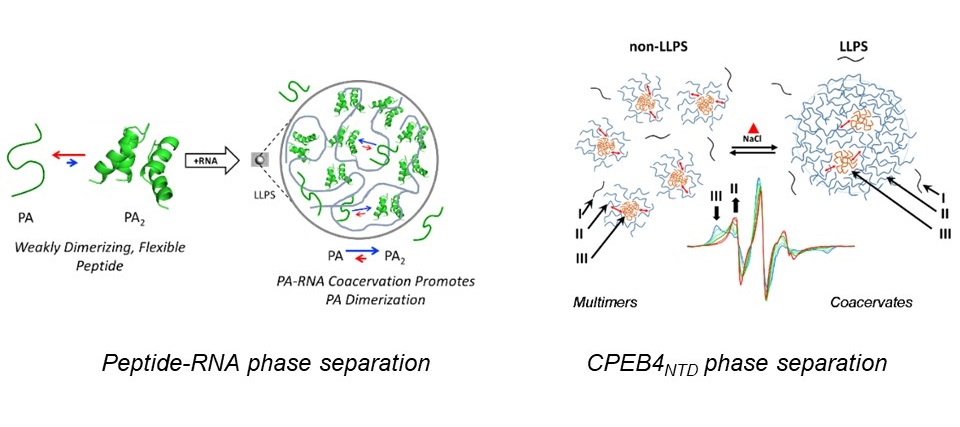
We work on two systems: In the first system, together with collaborators, we ask the question whether coacervates could have served as a unique testing ground for peptide oligomerization and if phase separating peptides could have been a resource for the construction of complex protein structures via common evolutionary processes, such as duplication and fusion. We study a primordial peptide with the helix-hairpin-helix (HhH) motif, an ancient and ubiquitous nucleic acid binding element, which forms peptide-RNA coacervates. Using EPR techniques combined with site-specific spin labeling of peptides containing one HhH motif, we explore its potential to dimerize and form the (HhH)2 fold to retain partial helical structure in solution, and the effect of RNA binding and the presence of LLPS on the dimers stability.
The second system is the low complexity N-terminal domain of cytoplasmic polyadenylation element binding-4 protein, CPEB4NTD, and its isoform CPEB4Δ4NTDwhich is depleted of an arginine rich 8 amino acid domain, referred to as Exon4,. These two proteins undergo LLPS with increasing temperature and they form highly stable nanometer-sized mulitmers which are precursors of the coacervates. This system supports the recent notion that the classical nucleation theory used to explain the formation of coacervates via nucleation of sparse monomeric molecules in a saturated solution does not always apply and pre-nucleation clusters in a saturated solution (two-step nucleation) is another mechanism. To understand the role of the multimers we employ optical microscopy, UV-vis extinction to follow macroscopic properties such as the LLPS transition temperature, dynamic light scattering (DLS) and atomic force microscopy (AFM) to determine the size of the multimers. This is combined with molecular level investigation by EPR spectroscopy, which provides the dynamic behavior of the constituents of the multimers and coacervates.
References
-
Peptide-RNA Coacervates as a Cradle for the Evolution of Folded Domains Seal M., Weil-Ktorza O., Despotovic D., Tawfik D., Levy K., Metanis N., Longo L. & Goldfarb D. (2022) Journal of the American Chemical Society. 144, 31. DOI: 10.1021/jacs.2c03819
-
Evolution of CPEB4 Dynamics Across its Liquid–Liquid Phase Separation Transition Seal M., Jash C., Jacob R., Feintuch A., Harel Y., Albeck S., Unger T. & Goldfarb D. (2021) The Journal of Physical Chemistry B. 125, 47, p. 12947-12957. DOI: 10.1021/acs.jpcb.1c06696


