Tzahor Lab
Cardiomyocyte cell-cycle control and dedifferentiation
Ischemic heart disease is the most common cardiac ailment, and the leading cause of death in the Western world today. We combine novel approaches to study cardiomyocyte (CM) renewal by promoting CM cell division and dedifferentiation, as a potential therapeutic strategy to cure the injured heart. We focus on various signaling strategies (e.g., NRG/ERBB signaling pathway), combined with attention to the microenvironment. We focus on the biophysical properties (e.g., matrix rigidity) of the heart microenvironment as well on biochemical contents of the ECM and ECM- associated molecules. Finally, we employ a novel state-of-the-art high-throughput screening platform to identify molecules that promote CM proliferation and to study their effects on cardiac regeneration in mice. We have identified several novel compounds which significantly increase the proliferation of adult CMs. Understanding how mature CMs can disassemble their sarcomeric architecture and re-enter the cell cycle, combined with the development of novel procedures that can facilitate CM dedifferentiation and proliferation, are major challenges facing current biomedical research. Our research into myogenesis and cardiogenesis in chick embryos demonstrates that the FGF-ERK signaling pathway plays an inhibitory role in myogenic differentiation. We now seek to understand the molecular and cellular underpinnings of this phenomenon using adult muscle stem cells.
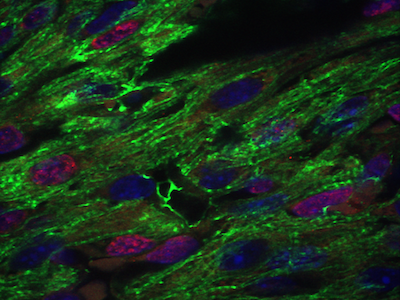
Cardiac and craniofacial development
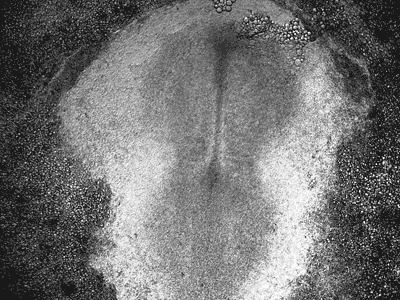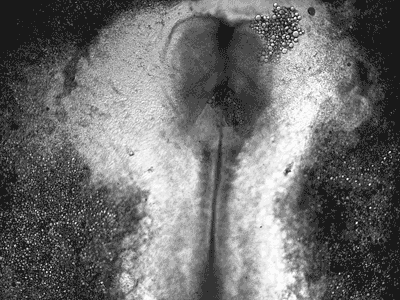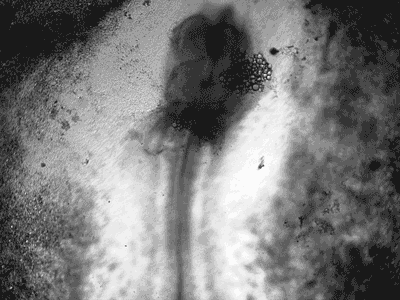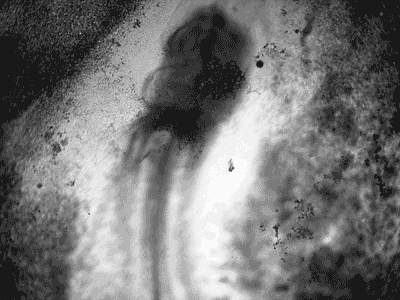
Manipulating tumorigenic processes to stimulate heart regeneration
Perhaps one of the most surprising and interesting aspects of the adult heart is the fact that cardiac tumors rarely develop. This implies that some factor/s within the heart, which we are keen to identify, resists tumorigenesis. Inducing oncogenic transformation of cardiac cells may help to understand the heart resistance to cancer. Unleashing cell cycle control is a hallmark of cancer; on the other hand, these same mechanisms could be very useful in promoting heart regeneration. Hence, another angle of our research focus on the pros and cons of cell-cycle re-entry, finding just the right balance between these two processes (regeneration and tumorigenesis).