Therapeutic window of antibody-based cancer immunotherapies
Despite the clinical success of antibody-based immune-therapeutics, notable limitations remain, including dose-limiting toxicity which can limit the clinical use of several of the most promising therapeutic antibodies. These toxicities may limit the maximum tolerated dose of the immunotherapies to a very low level, far below the optimal biological dose, and below an effective dose that can induce anti-tumor immunity. Our studies are focused on better understanding of the cellular and molecular pathways that lead to these toxicities and side effects. This allows us to develop new approaches and novel drug platforms to bypass these toxicities. Our approach to administer immunotherapies in a safe manner meets an urgent unmet medical need for implementation of promising immunotherapy targets towards clinical use.
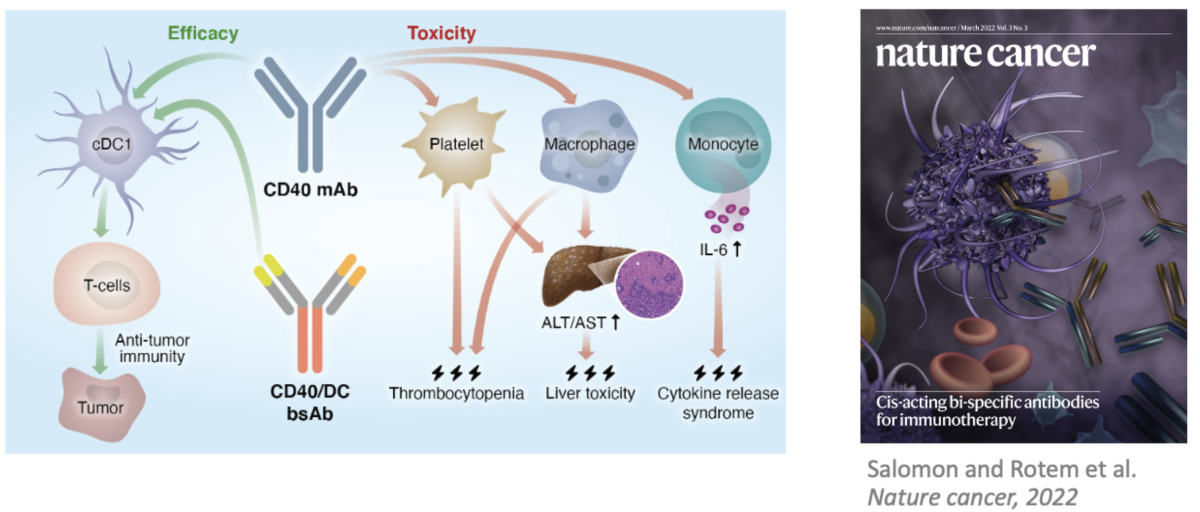
Related recent publication: Agonistic anti-CD40 antibodies are a potentially powerful approach for activation of the immune response to eradicate tumors. However, the translation of this approach to clinical practice has been substantially restricted due to the severe dose-limiting toxicities observed in multiple clinical trials. We demonstrated that conventional type 1 dendritic cells are essential for triggering antitumor immunity but not the toxicity of CD40 agonists, while macrophages, platelets and monocytes lead to toxic effects. Based on this knowledge, we designed and produced bispecific antibodies that preferentially target CD40 activation to dendritic cells, by coupling the CD40 agonist arm with dendritic cell-targeting arms. These bispecific reagents demonstrate a superior safety profile compared to their parental CD40 monospecific antibody, while triggering potent antitumor activity. We are developing such cell-selective bispecific agonistic antibodies as a drug platform to bypass the dose-limiting toxicities of anti-CD40, and of additional types of agonistic antibodies used for cancer immunotherapy.