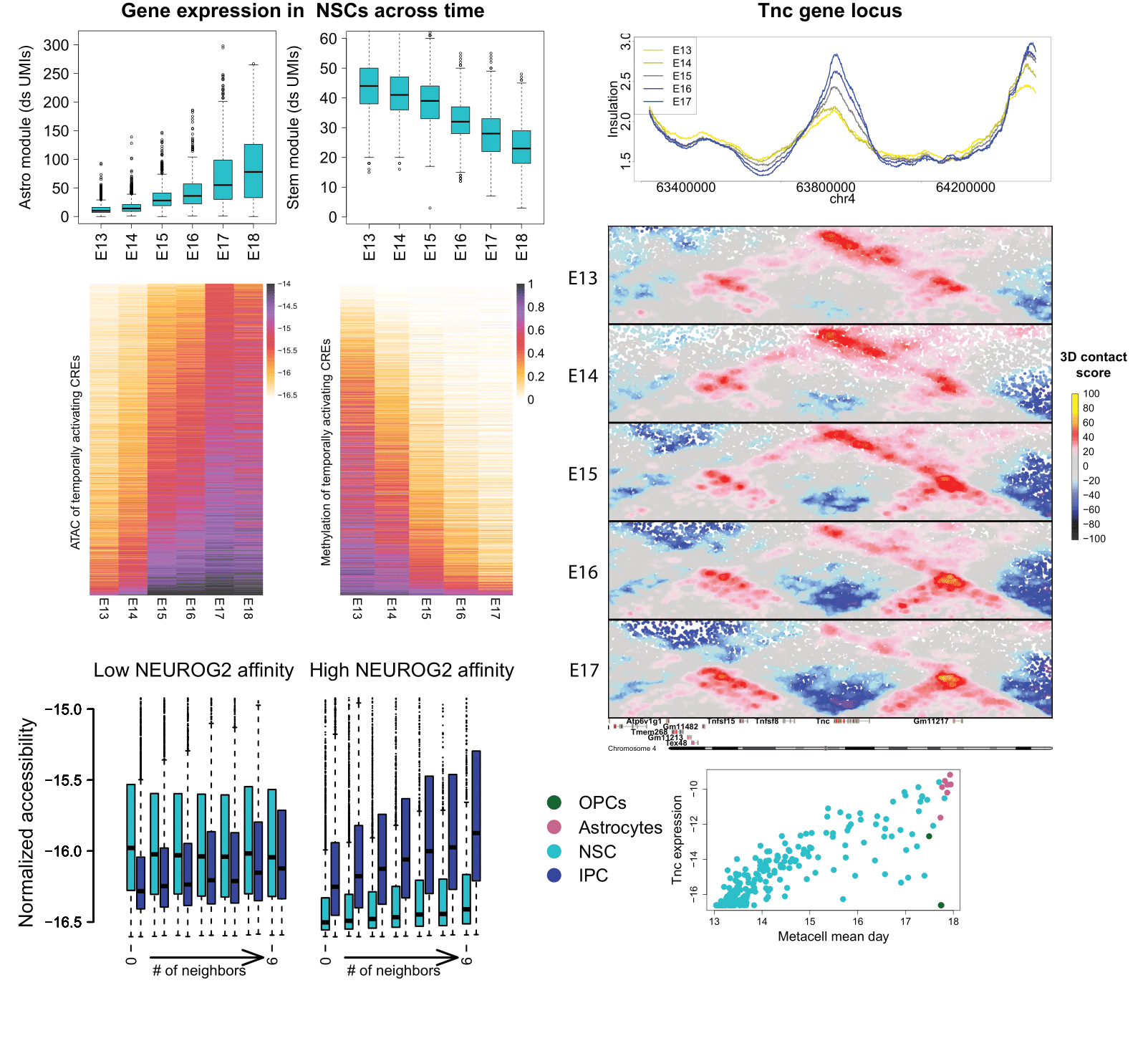
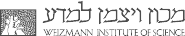The neural stem cell (NSC) population in the mouse cerebral cortex simultaneously proliferates and differentiates, by maintaining a stem cell population and producing a diverse array of neural and glial cell types. In collaboration with the Bonev lab, we generated and integrated gene expression, chromatin accessibility, methylation and chromatin topology data from days 13-18 of mouse embryonic development. We show that while neural stem cells gradually reduce their proliferation capacity, they also activate astrocyte-specific genes and cis-regulatory elements (CREs, also called enhancers), along with time-dependent demethylation and increased chromatin compaction. We also developed a machine-learning model, integrating sequence-based regression and epigenomic features, to predict the tendency of sequences to be accessible in either NSCs or a downstream progenitor state (IPCs), which was validated in a groundbreaking in-utero massively parallel report assay.