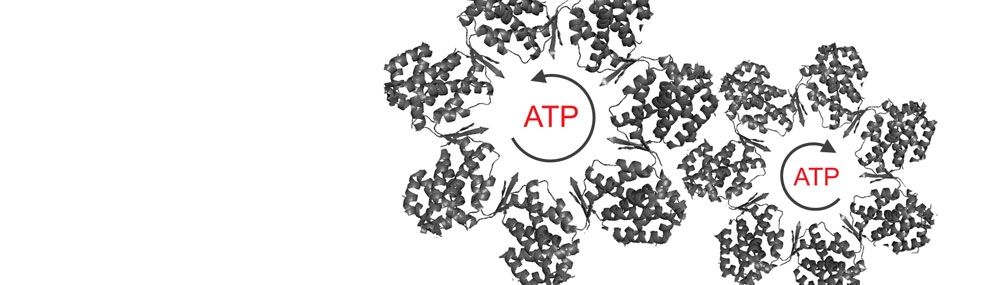
What is the relationship between allostery in chaperonins and their folding function?
Our group was the first to show a relationship between allostery in GroEL and GroEL-assisted protein folding rates (Yifrach and Horovitz, 2000). Using different cooperativity mutants of GroEL, we found a linear relationship between the folding rate of mouse dihydrofolate reductase (under conditions where its folding is GroEL-dependent but GroES-independent) and the rate and Hill coefficient of the T→R allosteric transition.