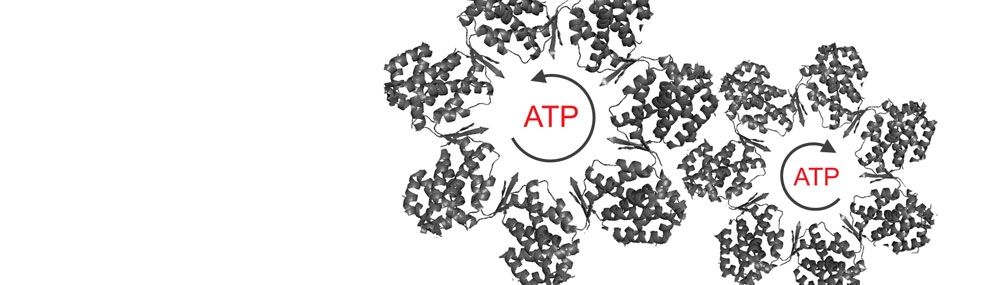
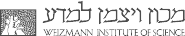The unique hetero-oligomeric structure of CCT/TRiC is thought to be responsible for many of the properties that distinguish it from GroEL such as the combinatorial nature of protein substrate binding by specific subunits and its sequential intra-ring allostery. In order to test the functional significance of the hetero-oligomeric structure, we generated a set of yeast strains with an identical mutation in each of the CCT/TRiC subunits, in turn, at a position that is conserved in all the subunits and is involved in ATP hydrolysis (Amit et al., 2010). In vivo analyses of these CCT/TRiC mutant yeast strains revealed surprisingly large phenotypic differences between them in, for example, growth rates, heat- and cold-sensitivities and morphology. These strains and mutant CCT/TRiC proteins are currently being used to unravel the substrate specificities of the different subunits of CCT/TRiC (Nadler-Holly et al., 2012) and to characterize its intra-ring sequential allosteric mechanism.
Additional structure-function studies of CCT/TRiC are also being carried out.