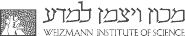Heterogeneous icing of super-cooled water (SCW):
Ice crystals melts at 0˚C at atmospheric conditions. However, water can be cooled below -40˚C without freezing. The ability to control the icing temperature of ice is of prime importance in the environmental sciences, for example for the glaciation of warm clouds for rain precipitation, in biology for the survival of cold-blooded animals in polar climates, in medicine for the preservation of tissues in transplantation, to mention but a few. Our research included the following systems: (in collaboration with L.Leiserowitz)
(i) Icing of SCW induced by amphiphilic alcohols at the air/water and within hydrophobic solvents/water interfaces.
(ii) The odd-even effect.
(iii) Induced icing within crevices of polar crystals.
Current research includes:
(iv) Electrofreezing as a chemical process as discovered on surfaces of pyroelectric crystals.
(v) Electrofreezing within electrolytic cells. The role played by “ice-makers” ions.
(in collaboration with I. Lubomirsky and D. Ehre)
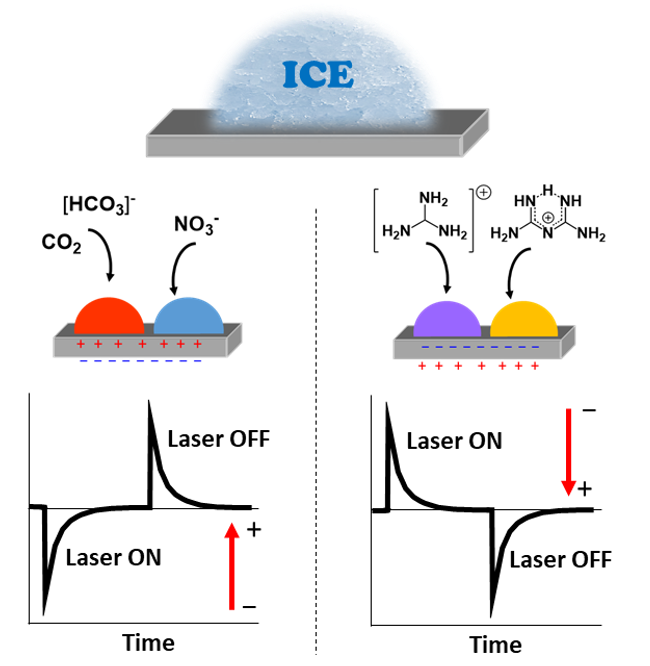
Electrofreezing on top of pyroelectric crystals