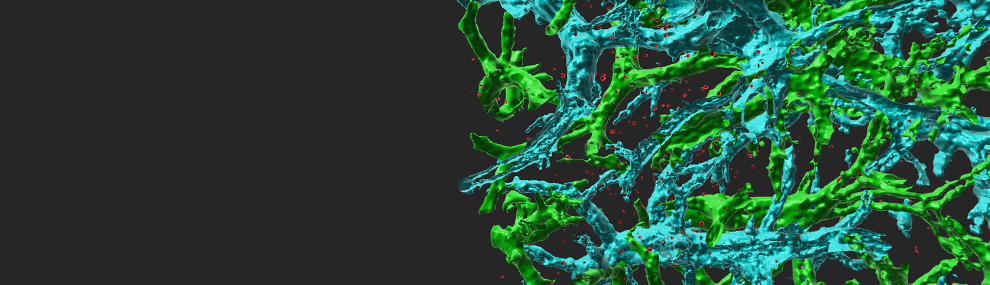
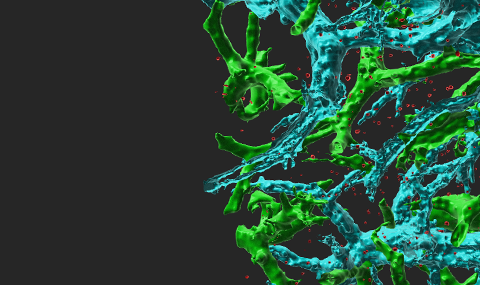
Endothelial-presented chemokines are critical for integrin-dependent adhesion and transendothelial migration (TEM) of naïve and memory lymphocytes. TH1 and Tc1 effector lymphocytes are special subsets of T cells that were recently activated by antigenic signals and underwent massive proliferation before they can exit the lymph nodes in which antigen was encountered. These cells were found by us to express adhesive integrins that can bypass chemokine signals and establish firm arrests on variably inflamed endothelial barriers. Nevertheless, the TEM of these lymphocytes strictly depend on Gi-signals and is promoted by multiple endothelial-derived inflammatory chemokines, even in the absence of outer endothelial surface exposure. Instead, TEM-promoting endothelial chemokines are stored in Golgi-derived vesicles that slide on microtubules (Movie 1), get docked on actin fibers beneath the plasma membranes (Figures 1,2), and are locally released within tight lymphocyte-endothelial synapses. Thus, effector T lymphocytes can cross inflamed barriers via a contact-guided consumption of intra-endothelial chemokines (Movie 3), in the absence of surface-deposited chemokines or extra-endothelial chemokine gradients (Figure 3). These results also suggest that endothelial cells can control, in a cell autonomous manner, through transcription and expression of adhesion molecules and chemotactic cytokines, all steps of leukocyte trafficking including, attachment, rolling, sticking, crawling and diapedesis (Scheme 1).
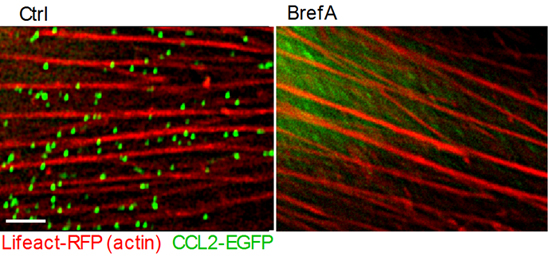
Figure 1 Vesicle-stored CCL2 drives effector lymphocyte transendothelial migration TNF-activated HUVEC were transiently transfected with CCL2-EGFP and the actin marker, Lifeact-mRFP. Transfected HUVEC were treated with medium or brefeldin A (BrefA) for 1 h and analyzed by live microscopy. Bar= 3 µm.
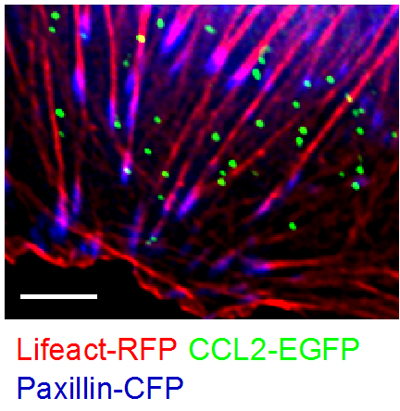
Figure 2 CCL2 vesicles docked on actin fibers beneath the plasma membrane promote effector lymphocyte TEM. TNF-HUVEC, transfected with CCL2-EGFP, Lifeact-mRFP and paxillin-CFP (blue), visualized by fluorescence microscopy.
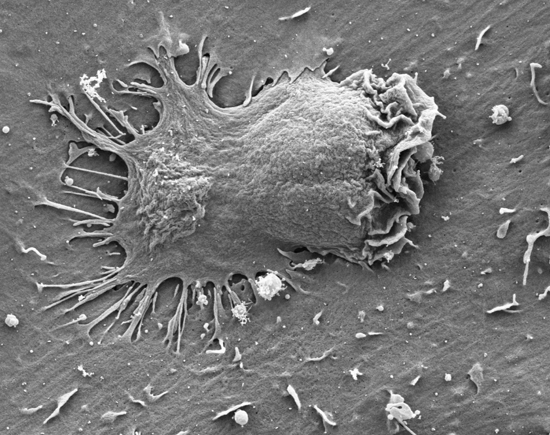
Figure 3 Transendothelial migration of effector lymphocytes mediated by intra-endothelial vesicle stores of endothelial produced chemokines. These endothelial produced cues get secreted into submicron contacts between the lymphocyte and the endothelial cell on which it spreads and promote formation of numerous ventral protrusions essential for lymphocyte crossing.
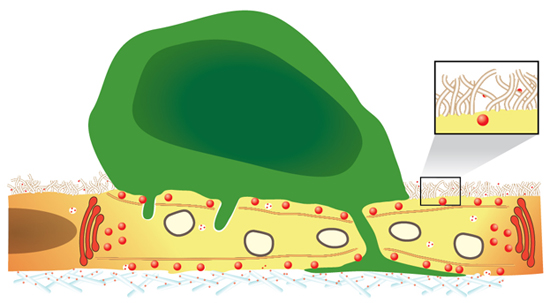
Scheme 1 Postulated roles for endothelial HS proteoglycans in chemokine immobilization on vascular and lymphatic endothelia To activate integrins on most rolling leukocytes, chemokines must be immobilized on HS GAGs of the endothelial glycocalyx (enlarged, inset). Nevertheless, effector T cells bypass this step and upon encounter of vesicle-stored inflammatory chemokines, readily protrude into endothelial cells and into paracellular junctions. These chemokine triggered protrusions develop into a large leading edge that integrates additional signals either from endothelial stored or HS GAG-immobilized chemokines within the ECM of the basement membrane.