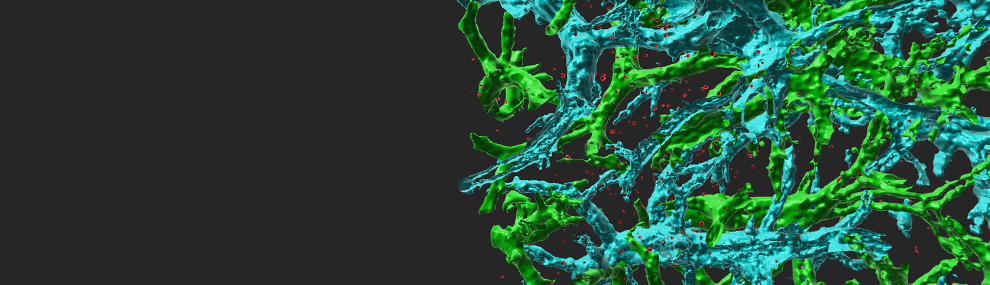
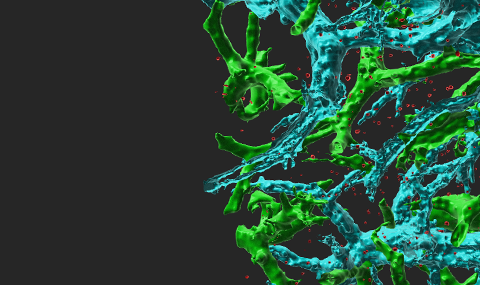
Lymphocyte stoppage on dendritic cells (DC) requires activation of LFA-1 by T cell receptor (TCR) signals, but the molecular basis of this activation is elusive. Using antibodies for specific LFA-1 conformations, we found that TCR activation in resting lymphocytes was insufficient to trigger LFA-1 extension or headpiece opening. However, TCR signals facilitate rapid conformational activation of LFA-1 by immobile ICAM-1, and promote lymphocyte spreading without requirement for cytosolic Ca2+. The T cell-DC synapse is mediated by numerous scattered focal dots of ICAM-1-rearranged high affinity LFA-1. The CCL21 chemokine dramatically accelerates LFA-1 responsiveness to TCR stop signals. Interestingly, T cells locomoting on immobilized CCL21 and CXCL12 cluster their LFA-1 in the leading edge and thereby prime it to rapid activation by TCR signals. Thus, promotile chemokines not only increase T cell scanning of DCs but sensitize LFA-1 to arrest these motile lymphocytes upon encounter of correct antigen (Scheme 1). The integrin adaptor, Kindlin-3 is critical for LFA-1 affinity triggering by TCR signals but dispensable for lymphocyte motility on chemokines. Our results suggest that TCR signals activate LFA-1 conditional to its occupancy by ICAM-1 within submicron focal dots. We suggest that these dots are the quantal adhesive units of the multi-focal lymphocyte-DC synapse (Figures 1,2). Notably, these adhesive units do not give rise to the invasive filopodia observed when the same T cells are spread on endothelial cells (Figure 3). The characteristic ring of LFA-1 observed in many in vitro studies of T-B synapses is probably a terminating supramolecular structure rather than the initial prerequisitory adhesive unit required for T cell stoppage on antigen presenting cells.
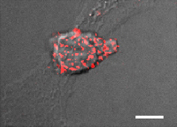
Figure 1 The quantal adhesive units of TCR stimulated T cells spread on DCs are scattered focal dots enriched with high affinity LFA-1. T cells were incubated with OKT3 (10 µg/ml) and labeled with a trace of a non blocking (Alexa 568 labeled) anti LFA-1 mAb. Lymphocytes were allowed to spread on prespread DCs for 5 min and fixed. A merge DIC/fluorescence image of LFA-1 in a representative T cell spread on a DC is shown. Bar, 3 µm.
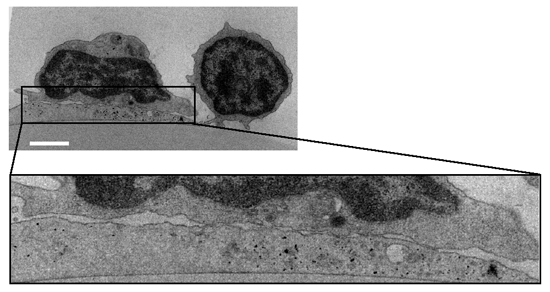
Figure 2 Transmission EM image of a representative OKT3-stimulated (10 µg/ml) normal T cell spread on a DC prespread on immobilized CCL21 As no Kindlin-3 null T cells could be recovered from the DC after fixation, similar fluorescence staining and EM microscopy could not be carried out for these T cells. Bar, 3 µm.
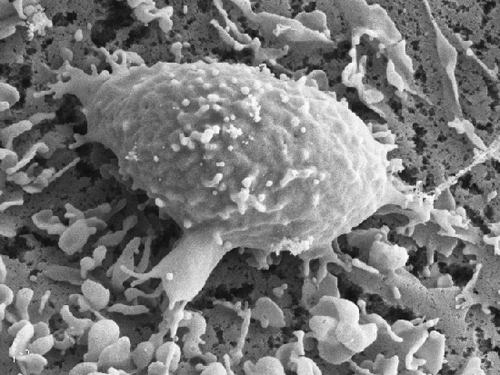
Figure 3 A SEM image of a representative OKT3 stimulated T cells spread on a DC prespread on immobilized CCL21 Bar, 10 µm. Note the numerous adhesive filopodia underneath the T cell body.

Scheme 1 A proposed model for LFA-1 conformational switches triggered during lymphocyte motility on CCL21 and by strong TCR signals encountered in vitro on 2D surfaces Resting PB T cells express globally inactive LFA-1 with low affinity to ICAM-1. During motility on the immobilized chemokine, LFA-1 molecules cluster at the leading edge of the polarized T cell, and a subset of these LFA-1 molecules undergo priming and partial headpiece activation depicted by opening of the b2 I domain, exposing the 327C epitope. This inside-out priming step is Kindlin-3 independent. The aL I domain on normal PB T cells remains in a closed conformation with low affinity to ICAM-1 and is therefore non-adhesive. When a concomitant TCR signal is encountered by a motile lymphocyte, the primed LFA-1 becomes hyper-responsive to an outside-in, ICAM-1 driven rearrangement event, which stabilizes the aL I domain in an open, high affinity conformation resulting in a firm LFA-1: ICAM-1 bond and highly stable contact with immobilized ICAM-1 (displayed on a bead or on a DC, not shown). This critical TCR stimulated outside-in LFA-1:ICAM-1 bond stabilization does not take place in Kindlin-3 deficient T cells. The magnitude of this mechanism is probably lower when ICAM-1 is presented by DCs in vivo possibly because cellular ICAM-1 is not properly anchored to the DC cytoskeleton.