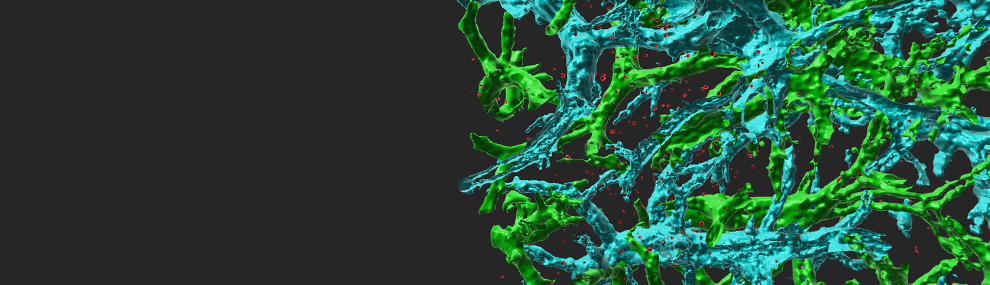
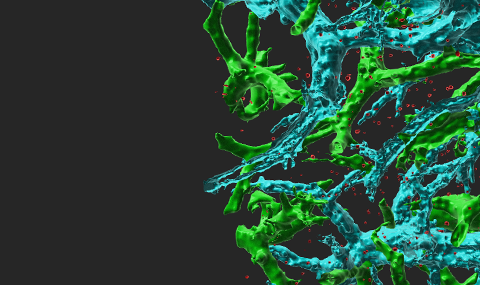
Leukocyte transendothelial migration (TEM) through inflamed endothelial cells (ECs) is initiated by leukocyte extension of a large leading edge under the endothelial barrier (basolateral lamellipodia) into which leukocytes squeeze their nuclei and thereby generate micron-sized gaps, primarily in between ECs (paracellular TEM). Our electron microscopy analysis suggests that ECs contain a dense cortical actin cytoskeleton which imposes a mechanical barrier for this dramatic squeezing process. Gap formation critical for leukocyte squeezing through multiple types of flat ECs and routes (paracellular or transcellular) involve leukocyte actin polymerization and contractility machineries that push forward and propel the leukocyte nucleus to exert outward forces on the EC cytoskeleton. Surprisingly, on the endothelial side, contractility machineries are not obligatory for gap opening by squeezing leukocyte nuclei. Our live imaging and biochemical manipulation of EC actin turnover suggest that the squeezing nuclei of leukocytes generate 4-5 micron wide gaps by collapsing thin EC actin filaments without rupturing the EC actin or vimentin bundles. We investigate how the differently shaped nuclei of neutrophils and T cells deform, squeeze and remodel these bundles as well as thinner actin filaments. Our models are based on inflamed microvascular ECs, the predominant type of ECs that support leukocyte TEM across post capillary venules at peripheral sites of inflammation. We seek to understand if and how nuclear stiffness restrict leukocyte TEM. We currently investigate whether leukocyte integrin traction forces generated at the front of transmigrating leukocytes coordinate the generation of leukocyte lamellipodia and help nuclear squeezing. These questions are addressed primarily in vitro in shear-flow based real time assays using phase contrast and fluorescence imaging as well as actin microscopy of differentially manipulated ECs and leukocytes. State-of-the-art correlative video microscopy and scanning electron microscopy approaches are also implemented to follow leukocyte-guided remodeling of endothelial cytoskeletal assemblies at nm resolution. The in vivo contribution of nuclear lamins to specific extravasation steps of leukocytes are studied in adoptively transferred, genetically manipulated leukocytes.
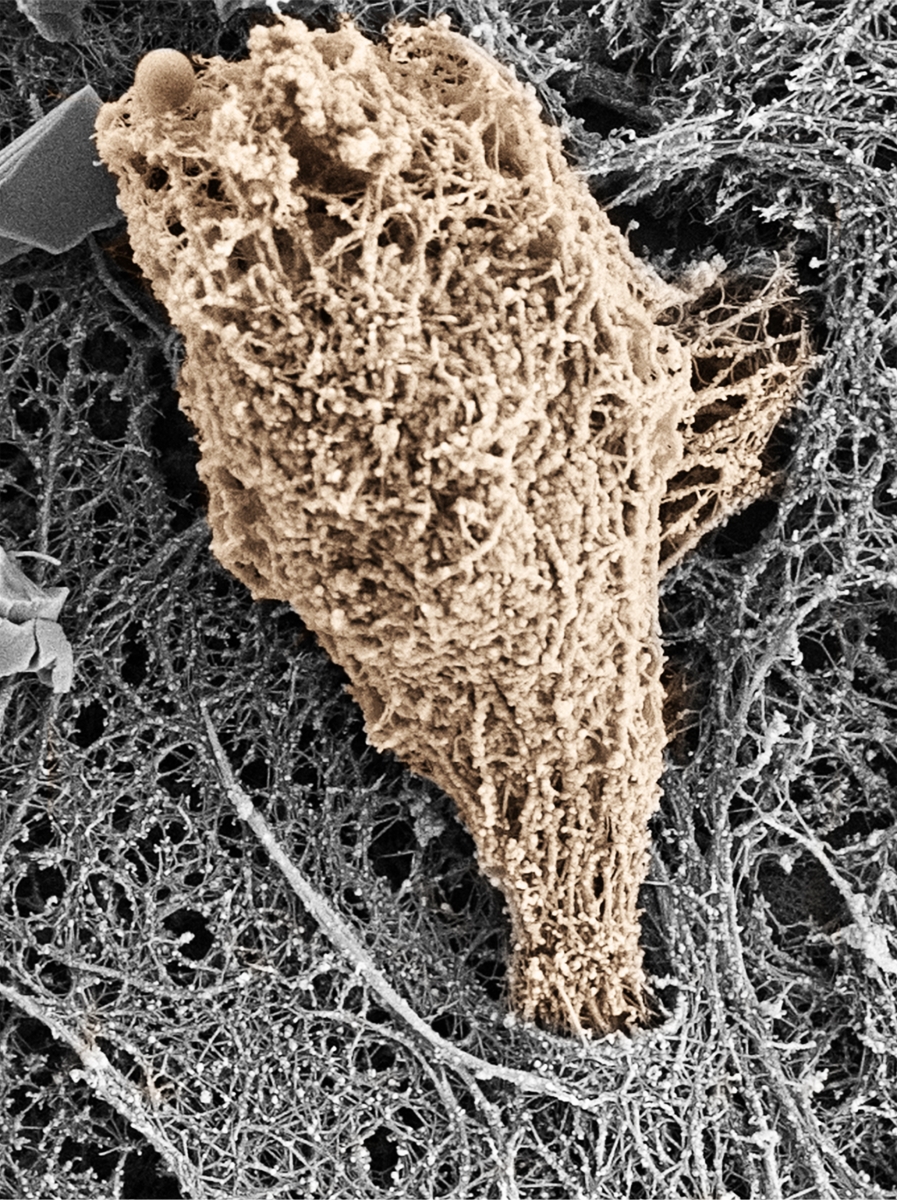
Effector T cell squeezing through inflamed IL-1β stimulated HDMECs. Several minutes after T cell perfusion into the flow chamber the lipid extraction and cytoskeleton-stabilizing solution was perfused into the flow chamber. The fixed sample was further processed for SEM and individual images were correlated to individual video recorded fields of view. The membrane stripped effector T cell depicted in this image is shaded in brown for clarity. Initial protrusion of this lymphocyte is sent into an endothelial cell during transcellular migration of the T cell through the endothelial cell (grey).