Nuclear stiffness is controlled by the ratio between different lamins, major intermediate filament constituents of the nuclear lamina. Transendothelial migration of lymphocytes and neutrophils is associated with the ability of their deformable nuclei to displace endothelial cytoskeletal barriers. Lamin A is a key intermediate filament component of the nuclear lamina which regulates nuclear stiffness. To determine if the low lamin A expression kept by most leukocyte nuclei is critical for their exceptional squeezing ability through endothelial barriers, we overexpressed this protein in leukocytes and assessed how changes in nuclear stiffness affect leukocyte squeezing through different barriers. Surprisingly, leukocytes with stiff nuclear lamina were found by us to successfully complete paracellular TEM across inflamed endothelial monolayers, albeit with a small delay in nuclear squeezing into their sub-endothelial pseudopodia (Movie 1). In contrast, leukocyte motility through collagen I barriers was dramatically delayed by lamin A overexpression due to a failure of lamin A high nuclei to translocate into the pseudopodia of the leukocytes. Collectively our data predict that leukocytes maintain a low lamin A content in their nuclear lamina in order to optimize squeezing through extracellular barriers but can tolerate high lamin A content when crossing the highly adaptable barriers presented by the endothelial cytoskeleton.
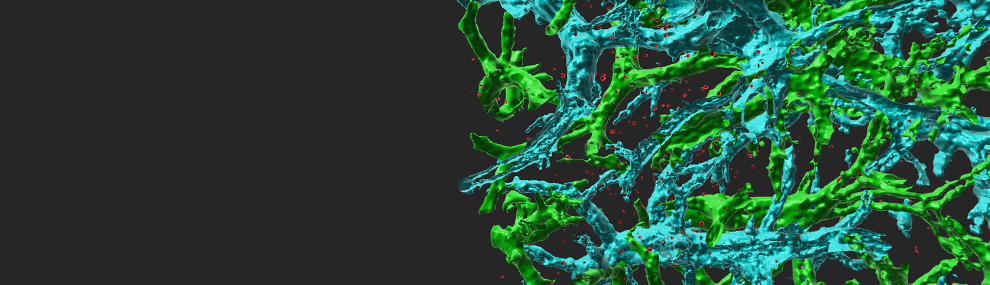
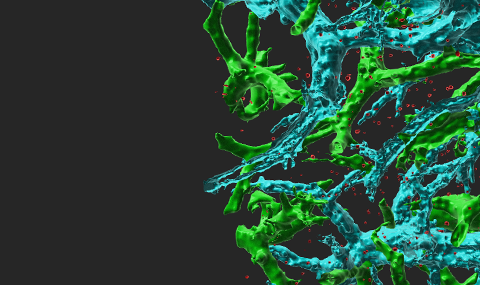
Metastasis is responsible for > 90% of cancer patient mortality. Metastasis involves two critical barrier crossing events, intravasation into blood vessels and extravasation from blood vessels at target organs. Extravasation to the lung parenchyma occurs in the pulmonary vasculature, an extensive network of relatively impermeable capillaries. Since metastasis to other organs such as the bone marrow and the liver occurs via much more permeable sinusoids we predict that only a small subset of circulating breast cancer cells entering the pulmonary capillary bed can overcome this specialized vascular barrier. We have recently established a new in vitro system in which tumor cell TEM is analyzed in real time (Movie 2). Tumor cell crossing of endothelial barriers involves entirely different machineries than leukocyte TEM and is much slower (hrs vs. minutes in the case of multiple types of leukocytes). Our recent in vitro findings suggest that a subset of metastatic cells leave behind their stiff nuclei while their detached pseudopodia rapidly cross endothelial barriers. We therefore assess the possibility that tumor transendothelial migration involves the transient softening of their otherwise stiff nuclei, without which this large organelle fails to pass through the tight pulmonary vascular bed. We also hypothesize that this transient softening of the nuclear lamina occurs both before and after intravasation of highly invasive tumor cells into the blood vessels surrounding the primary tumor. The A-type lamins are expressed at very low levels compared with the B-type lamins during early embryonic development and their expression increases with cell differentiation along with nuclear stiffening. Surprisingly, reduction in the stiffness of the nuclear lamina introduced by silencing of lamin A and C, its spliced isoform lamin facilitates cancer cell migration through rigid mores but not cancer cell TEM or extravasation out of lung vessels. We also follow how reduced lamin A/C content within the nuclear lamina affect nuclear rupture events and premature apoptosis after extravasation into the lung parenchyma. Using shRNA mediated silencing of lamins A/C and adoptive transfers of fluorescent lines into syngeneic recipients bearing primary tumors, analysis of cancer cell extravasation by light sheet microscopy-aided 3D imaging of whole lungs (Movies 3,4,5), and real time intravital microscopy of differently mutated cells entering the lung parenchyma, we follow if transcriptional lamin A downregulation equally facilitate breast cancer intravasation and extravasation across lung capillaries and whether altering lamin expression also change epigenetic states and cell cycle progression of different cancer cells. Our study is likely to identify if and how subsets of metastatic cells remodel their nuclei in order to proliferate, migrate and extravasate from lung capillaries.