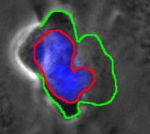
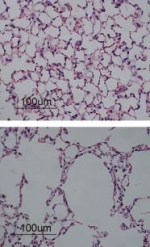
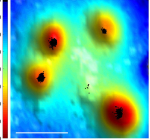
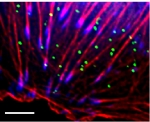
Introduction
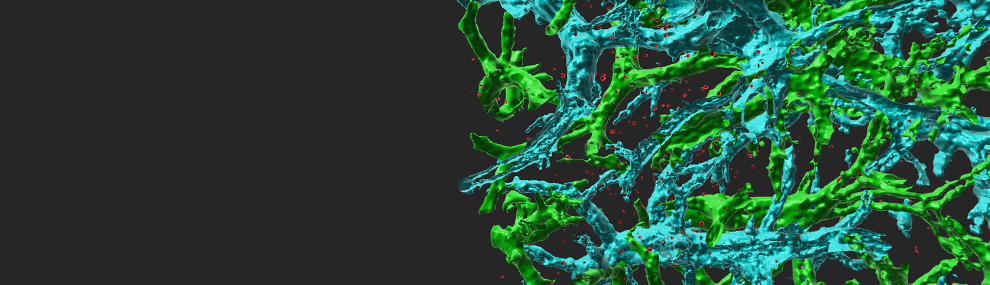
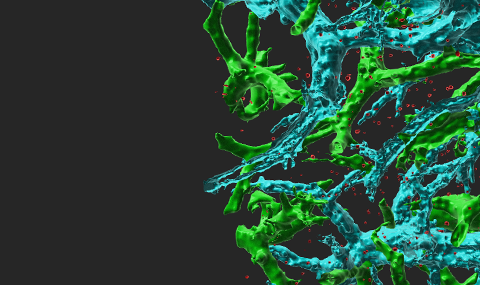
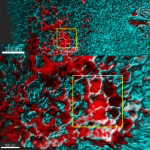
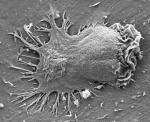
Circulating immune cells and hematopoietic progenitors must exit blood vessels near specific target sites of injury, inflammation or tissue repair. The vessel wall at these sites displays specific combinations of traffic signals in the form of adhesion molecules (selectins, integrins) and chemotactic cytokines (chemokines) which operate in sequence to recruit only specific circulating subsets with proper receptors to these signals. As these processes take place under shear stress, these traffic molecules have evolved to operate under specialized kinetic and mechanical contexts.