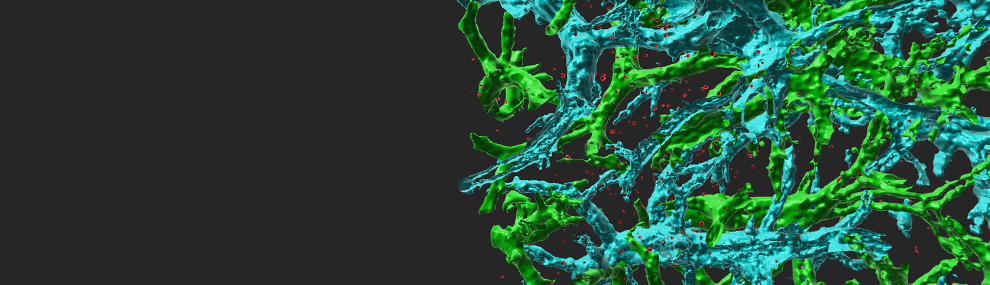
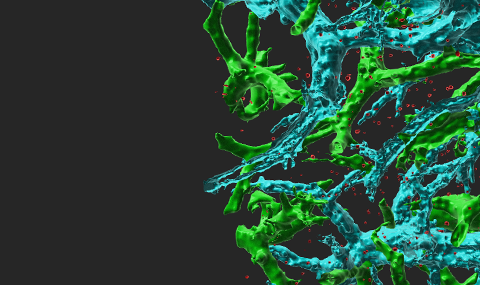
Circulating immune cells and hematopoietic progenitors must exit blood vessels near specific target sites of injury, inflammation or tissue repair. The vessel wall at these sites displays specific combinations of traffic signals in the form of adhesion molecules (selectins, integrins) and chemotactic cytokines (chemokines) which operate in sequence to recruit only specific circulating subsets with proper receptors to these signals (Scheme 1). As these processes take place under shear stress, these traffic molecules have evolved to operate under specialized kinetic and mechanical contexts. Using special flow chambers which simulate blood flow in the circulation (Scheme 2) we attempt to dissect how these molecules and their cytoplasmic associations with the cell cytoskeleton mediate cell adhesion and exit through blood vessels. Videomicroscopy of immune cells interacting with vessel cells (Movie 1 and Movie 2) and subcellular staining of both adhesion receptors and their specific cytoplasmic regulators allow us to follow spatially and temporally how these molecules mediate lymphocyte exit across the blood vessel walls and how antigenic signals are integrated into stoppage of extravascular lymphocytes on dendritic cells and macrophages. This information is key for the development and implementation of therapeutic tools to fight chronic inflammatory disorders, autoimmunity, allergy, heart injury, atherosclerosis, organ rejection and metastasis.
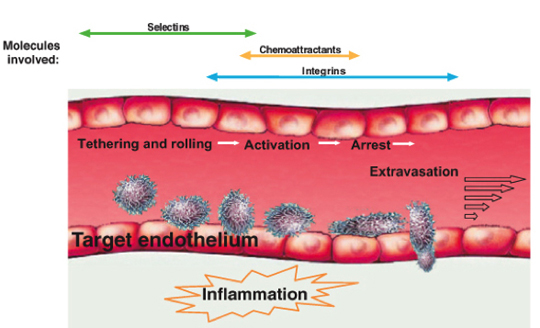
Scheme 1 A multistep model for leukocyte and stem cell recruitment at target vessel walls. Selectins, chemoattractants (chemokines) and integrins coordinate in capturing a circulating leukocyte to the vessel wall at specific sites and in generating resistance to detachment by the blood shear forces. Further leukocyte exposure to apical endothelial chemokines and shear flow trigger leukocyte transmigration through the endothelial barrier.

Scheme 2 The in vitro flow chamber apparatus and attached videomicroscopy setup used in the lab for recording real time adhesion and migration of immune cells under conditions simulating physiological blood flow
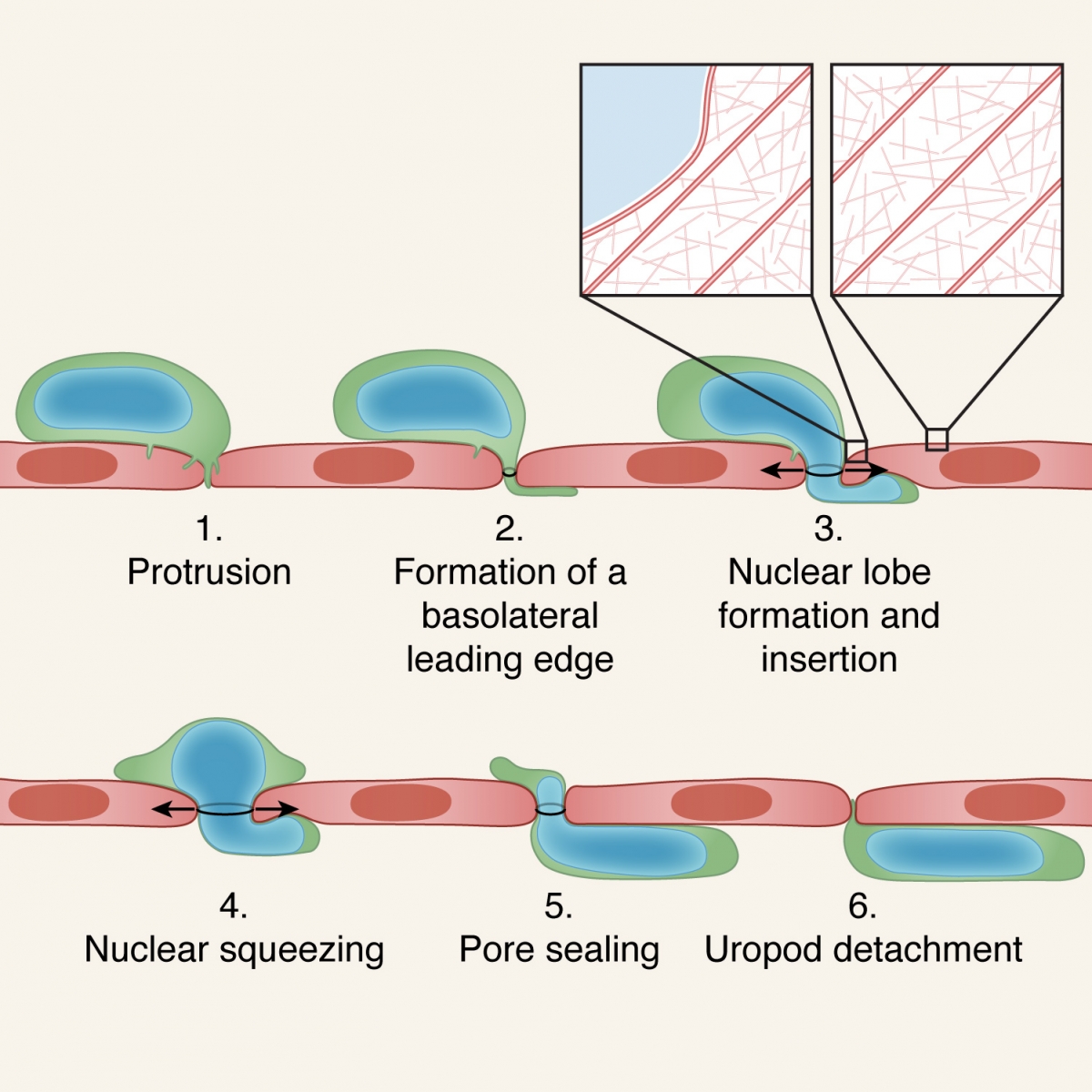
A 6 step model for leukocyte transmigration. The leukocyte protrudes via multiple invasive filopodia which can merge and develop into a sub-endothelial leading edge (steps 1,2). (pseudopodium). The deformable nucleus generates a lobe that generates a small gap by displacing and collapsing different actin cytoskeletal components within the neighboring endothelial cells. The nuclear lobe also gets inserted into this thin pseudopodium and lifts the endothelial cell above its basement membrane (step 3). The nucleus gets squeezed further enlarging the gap (step 4). The entire nucleus is inserted underneath the endothelial layer. The uropod of the leukocyte detaches from the apical endothelial surface (step 6). For simplicity only paracellular leukocyte migration is shown. In the case of polymorphonuclear leukocytes (e.g., neutrophils), preexisting nuclear lobes get serially inserted. For transcellular migration, leukocytes use their invasive filopodia to generate a pore through which their entire nucleus gets squeezed via its individual lobes.