Cells receive sensory input from neighboring cells that allows them to rapidly respond to changes in their surrounding environment. This can occur via secreted signaling molecules that bind to cell surface receptors and elicit downstream signaling events that lead to changes in cell physiology. While much information is known about cell-cell communication via signaling molecules, much less is known about whether cells transfer information via the exchange of RNA. Despite this paucity of knowledge, most extracellular fluids contain RNA molecules, mainly within secreted nanovesicles, although free ribonucleoprotein complexes have also been identified. Most of the extracellular RNA is composed of small RNAs (e.g. miRNA, tRNA and mRNA fragments). Thus, the horizontal transfer of RNA may be yet another means for cells to exchange information and, perhaps, constitutes part of the mechanism by which cells like neurons can reach meters in length and still respond rapidly to injury or changes in synaptic signaling (i.e. via cell-cell transfer of RNAs between myelinating cells and axons).
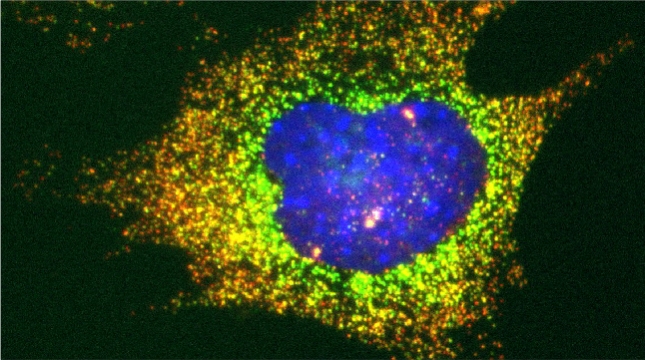
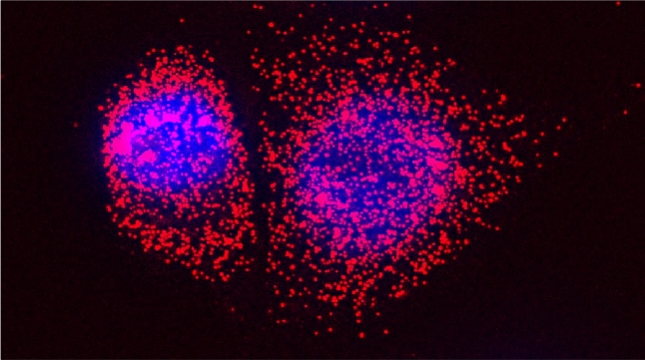
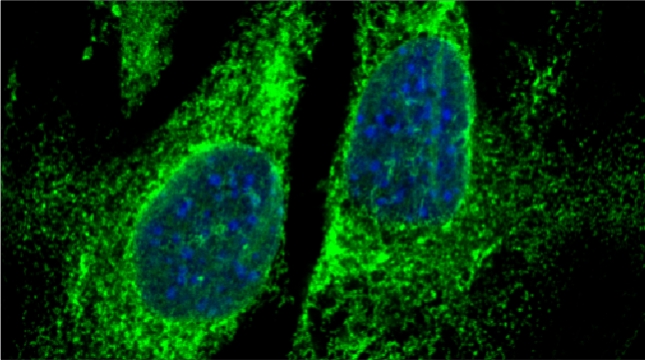
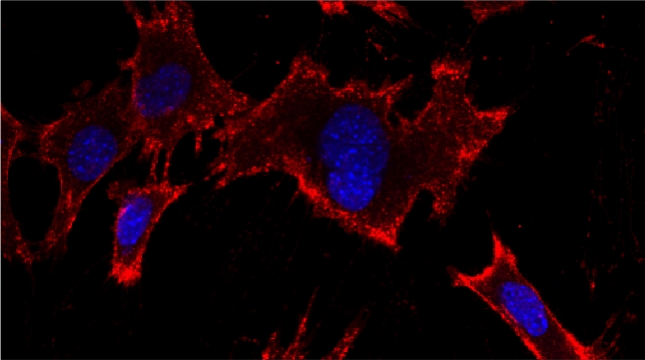
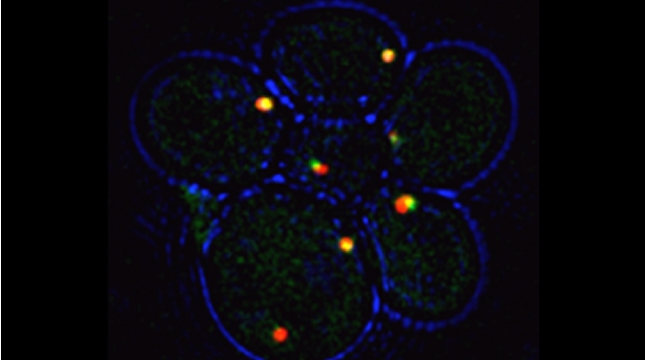
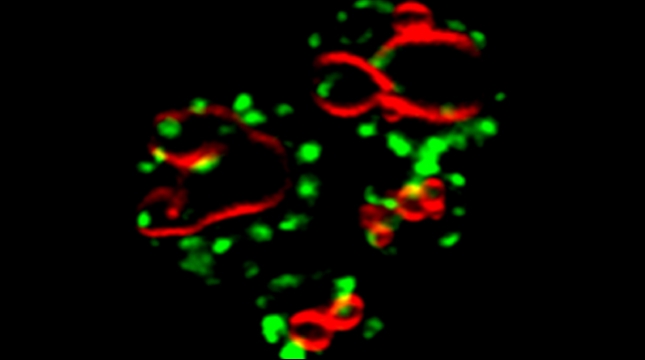
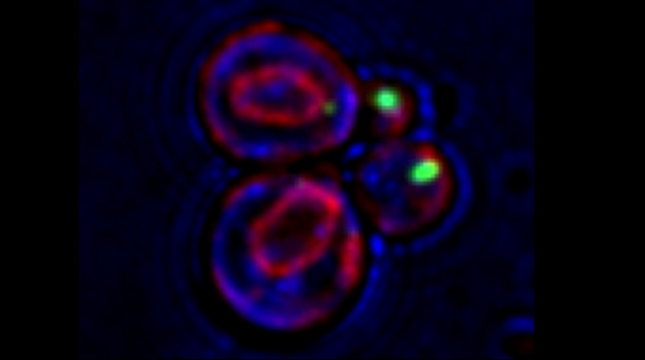
We examined whether cultured cells, such as mouse fibroblasts or cancer cell-lines, can transfer mRNAs between themselves and, thereby, possibly affect the cellular physiology of neighboring cells. In collaboration with the group of Robert Singer (Albert Einstein College of Medicine, Bronx, NY) and Arjun Raj (University of Pennsylvania), we have shown that donor cells can transfer mRNA to naïve acceptor cells [Haimovich et al 2017]. By using single molecule fluorescence in situ hybridization [Haimovich & Gerst 2018] and single molecule live imaging procedures we can show that mRNA molecules undergo horizontal cell transfer. Surprisingly, this is mediated by a contact dependent mechanism called tunneling (membrane) nanotubes, and not by extracellular mRNA transfer. Furthermore, we found that the transferred mRNA is packaged in a protein "shell" of yet unknown composition [Haimovich & Gerst 2019].
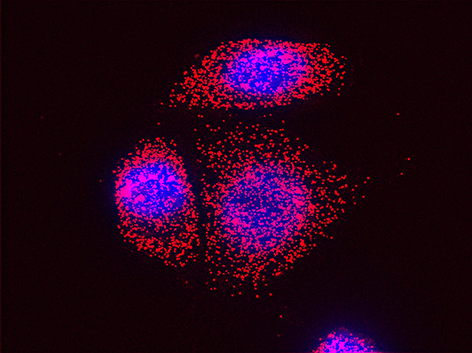
We believe that this phenomenon (reviewed in [Haimovich et. al., 2020]) will prove to be a common mechanism for cells to respond to and affect local changes in their local environment. We recently identified the entire "transferrome" using a protocol we developed in the lab [Dasgupta & Gerst, 2020]. We found that mRNAs and lncRNAs of almost all human genes can transfer, in a direct correlation to gene expression level [Dasgupta et al. 2023].
We further found that transfered mRNAs are being translated in acceptor cells, and cen elicit a physiological change in these cells. thus, mRNA transfer can complement a lack of a peroxisomal biogenesis protein. In addition, we developed a system to track this phenomena using CRE, which will allow us to further dissect this mecnaism both in vitro and in vivo [Haimovich et al. 2024].
We continue to study the molecular mechanism that mediates and regulates nanotubular mRNA transfer, and the physiological consequences of this process. We are curerntly developing mouse models to study this process in vivo as a form of RNA therapeutics.