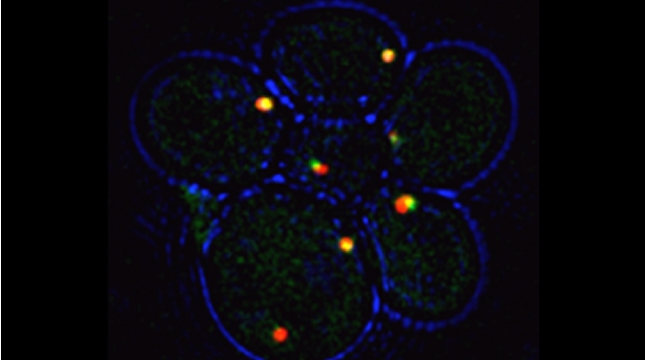
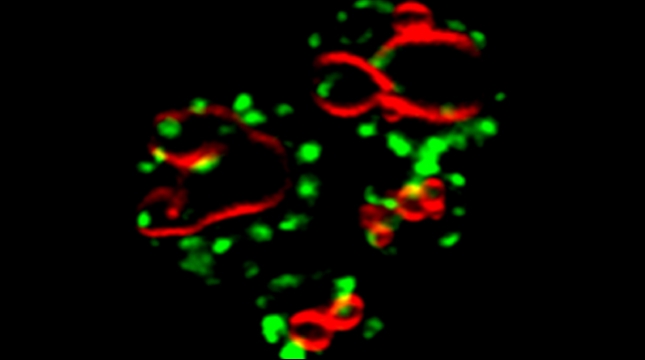
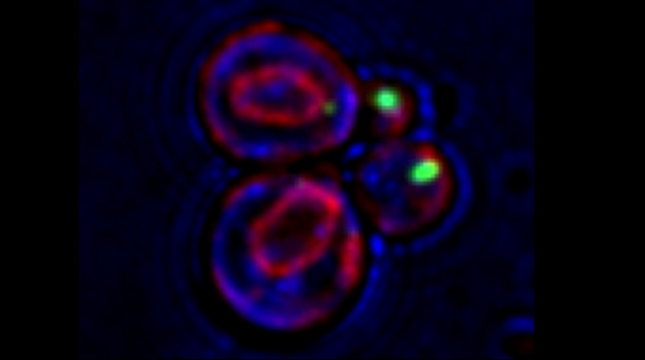
Earlier work in the lab identified proteins that bind to exo/endocytic v-SNAREs of the secretory pathway [Protopopov et al 1993, Gurunathan et al 2000], including Btn2 [Kama et al 2007], Gcs1 [Robinson et al 2006], and Ddi1 [Lustgarten and Gerst 1999, Marash and Gerst, 2003, Gabriely et al 2008]. Ongoing work on Ddi1, which is an ubiquitin receptor of the UBL-UBA family (also known as ubiquilins), shows that it associates with the Cdc48 AAA ATPase. While ubiquilins and Cdc48 are known to confer ER-associated degradation of proteins (ERAD), we find that Ddi1 is not involved in ERAD, but that it still acts with Cdc48 to confer anterograde vacuolar protein transport. We show that Ddi1 and the Cdc48-Ufd1-Npl4 complex regulate the sorting of specific proteins into the multivesicular body pathway by converting aggregated (oligomerized) substrates into import-competent monomers [Kama et al 2018]. Interestingly, both mammalian Cdc48 (p97/VCP) and the ubiquilins are major risk-associated groups for both amytrophic lateral sclerosis and Alzheimer’s disease. Thus, we are using yeast as a model to study the role of Cdc48 and ubiquilins in human proteostasis disorders.
Jeff Gerst's lab mRNA and Protein Trafficking