The Lightsheet enables fast and gentle fluorescence 3D imaging of large, live, and cleared samples with minimal photobleaching.
The Lattice-Lightsheet enables 3D fluorescence live cell imaging with minimal phototoxicity.
Multiphoton based on scanning-confocal. Suitable for imaging of thick samples and Second Harmonic Generation cell cultures, plants, tissue sections, or thicker samples.
Suitable for fast imaging of fixed tissue sections and cultured cells labeled with fluorescent or visible (color) markers.
Fully integrated high-end automated live cell imaging system that enables high sensitivity and focus stability in live-cell experiments (imaging period: days).
Offers fast and sensitive 3D imaging, utilizing WF imaging, confocal imaging with 2 different pinhole sizes (40µm for higher contrast/25 µm for higher resolution), or TIRF (total internal reflection) imaging.
STED (Stimulated Emission Depletion) mode that offers a resolution of up to 30nm lateral and ~100nm axial in 3D. Combination of white light pulsed laser (470nm-647nm) for excitation and gated spectral detection. Provides an excellent signal with full freedom in the choice of fluorophores at the visible range. The system also enables fluorescence lifetime measurements (FLIM) that determine how long a fluorophore remains on average in its excited state.
Nano live 3D cell explorer is a holo-tomographic microscope that measures the quantitative refractive index of cell organelles. Live cells can be imaged in 3D without stains, fixation, or phototoxicity.
A multiplexed imaging system, based on automated iterative imaging cycles of antibodies that are conjugated to oligonucleotides. The PhenoCycler-Fusion system enables the visualization of a large number of markers (up to 40) on a single tissue section.
Cell stretching instruments for biomimetic experiments. The system can be connected to an inverted Leica DMI8 Wide-Field and equipped with an environmental control unit (Temperature & CO2) for live imaging, or as a standalone for endpoint experiments.
Cell Observatory public- provides more information and links to additional training material.
Bioimaging@WIS -The website highlights the range of services and technologies available, which are open to any WIS research group.
Moross Integrated Cancer Center (MICC) -The de Picciotto Cancer Cell Observatory in Memory of Wolfgang and Ruth Lesser offers advanced imaging technologies for the in-depth and high-resolution analysis of cancer cells.
Short video bout the unit:
Imaging Systems

Light Sheet -
Lavision “Ultra Microscope”
Suitable for morphological studies of large samples (max 10*10*50 mm) cleared using water or organic solvents (RI:1.33-1.56). Provides fast 3D imaging with a Z resolution of ~5µm.
- a. Objectives:
- 1. Zoom Body (0.63 X-6.3X) WD 6mm (depends on the dipping cup). Equipped with an Olympus MVPLAPO 2X/0.5
Final magnification (1.3X – 12.6)
2. Infinity corrected single lens
LVMI-fluor 4X/0.3 (Zoom optional to 8X) WD 5.7mm
3. 1.3 air/dipping for overview
b. sCMOS at 16bit
c. Excitation/Emission filters:
|
Excitation filter |
Emission Filter |
|
470\40 |
525\50 |
|
514\10 |
595\40 |
|
545\25 |
595\40 |
|
560\40 |
630\75 |
|
640\30 |
690\50 |
|
710\75 |
810\90 |
 Lightsheet Z1 - Zeiss
Lightsheet Z1 - Zeiss
- Suitable for live (morphogenesis/embryogenesis, 3D culturs/ spheroids) fixed, or water based cleared (CLARITY/ Scale /Pact) whole tissues.
- Excitation objectives: 5X and 10X
- Detection objectives:
- Air: 5X
- Water: 10X-20X-40X
- Clearing: 20X clearing for 1.45 Refractive index / 20X clearing for 1.38 Refractive index
- Multiview imaging for maximal 3D resolution, multiposition imaging
- Combines environmental control for prolonged imaging with minimal perturbation.
- d. Emission filter combinations covering fluorophores excited by the 405-633
e. CCD at 16it
ZEISS Lattice Lightsheet 7

Suitable for cell culture, embryos, and 3D cell culture on coverglass No1.5 (170um thickness).
Enables extremely fast fluorescent image acquisition with minimal photodamage.
- A dual-camera setup allows one to acquire 2 channels simultaneously
- Orca Fusion (GenIII sCMOS sensor with liquid cooling) x2
Resolution is comparable with that on spinning disk confocal at 40x1.1 water immersion lens.
Suitable sample formats are that for inverted microscope: glass slide, multiwell plate, and 35mm Petri dishes, with some limitations.
Sample incubation allows heating, as well as humidity, CO2, and O2 control
Spectral flexibility
- Excitation: 488, 561, 640 nm laser
- Beam Splitters: 100% transmission, LP 565, LP 640
- Detection filter wheel; detection up to 900 nm
Automation
- Multidimensional acquisition (timelapse, multi-position, multichannel, z-stack macro)
- Auto immersion, autofocus adjustment (slow)
- Additional automation modules in ZEN software
- Postprocessing (deskew, 3D visualization, and more)
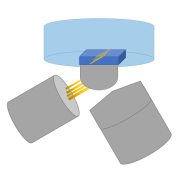

Leica SP8 confocal-2Photon (Upright)
Suitable for cell cultures, plants, tissue sections, or thicker samples. Enables Z compensation for even illumination.
-
-
- Excellent spectral separation and high sensitivity, combining Acuso-Optical-Tunable-Filter, spectral detection, and 1 PMT and 2 HyD internal detectors. HyD is characterized by high sensitivity and low noise detection.
- Chameleon Vision II coherent tunable laser, combined with 2 sensitive HyD external detectors (NDD) enables
- Second-Harmonic-Generation detection in 3D and 4D
- Objectives:
- HC PL FLUOTAR 10x/0.30 (air)
- HC PL APO 20x/0.75 CS2 (air)
- HC FLUOTAR L 25x/0.95 W VIS (water dipping)
- HC PL APO 40x/1.10 W CORR (water immersion)
- HC PL APO 63x/1.40 OIL CS2 (oil immersion)
- HC FLUOTAR L 25x/1.00 IMM Leica motCORR - ne=1.457 (for cleared samples)
- Large fields of view acquired in fast scanning mode, enable accurate navigation within the sample during high-resolution imaging
- Environmental control enables live imaging for longer periods, combined with a fast resonant scanner, sensitive detectors, and software solutions for lower phototoxicity
- LASX software pack, including integrated Deconvolution module
- Variety of optional sample holders for increased imaging stability of cleared or expanded sample
-

Leica DMI8 Wide-Field (Inverted)
- Suitable for fast imaging of fixed tissue cells, thin tissue sections, or thin plant sections.
- The motorized stage for large, accurate XY movements. Offers full navigation within the sample.
- High-speed autofocus allows an efficient 3D view of large areas
- 2 cameras optional: Leica DFC7000GT monochromatic, triggered camera (for fluorescent imaging) and DFC7000 T color camera
- Several transmission modes: Bright Field, Differential-Interference-Contrast (DIC) imaging, Phase imaging
- Objectives:
- 5X/0.12 dry
- 10X/0.25 dry
- 20X/ 0.4 dry
- 20X/ 0.8 dry
- 40X/0.6 dry
- 100X/ 1.4 oil
- Equipped with 2 separate filters for excitation and emission wavelengths, therefore allowing different combinations of excitation (402-488-561-561-638-100%) and emission (450/525/600/700/100%).
f. LASX software pack for analysis

Cell Discoverer 7-Zeiss (inverted)
Fully integrated high-end automated live cell imaging system that enables high sensitivity and focus stability in live-cell experiments (imaging period: days)
-
-
- Allows automated recognition of single plate/multi-well plates and chamber slide`s parameters: material (glass/plastic), thickness, skirt height (for maximal high-res lateral view)
- Objectives:
*effective (optical) zoom 0.5X-1X-2X
ZEISS Plan-APOCHROMAT:- 5x / 0.35 (Working distance 5.10 mm)
- 20x / 0.7 Autocorr Objective (Working distance 2.20 mm)
- 20x / 0.95 Autocorr Objective (Working distance 0.76 mm)
- 50x / 1.2 W Autocorr and Autoimmersion Objective (Working distance 0.84 mm)
*Automated water injection/removal allows smooth shift between magnifications during experiments
- Combination of hardware and software-based focus strategies allow fast focus determination and stability over long periods
- Excitation LEDs : 385, 420, 470, 511, 567, 590, 625. The system is equipped with high-efficiency multibandpass filter sets. Other contrast options include: bright field, DIC, and phase gradient
- Two cameras: Zeiss Axiocam 506 mono and s CMOS 702
- Environmental control: temperature range within sample chamber: 30 – 45 °C or cooling unit controls the temperature of the top plate of sample chamber with a temperature range of 14 – 28 °C.
- An O2-control device for Hypoxia experiments, done by displacement with N2 within the sample chamber.
- Possibility for smart experimental design: Use Python scripts to customize and automate your workflows.
-
• Integrate external image analysis applications into your workflows.
• Exchange image data with external programs like ImageJ, Fiji, MATLAB, KNIME, or Python.
• Use feedback for smart experiments.

Andor Dragonfly on a Leica DMi8 microscope (inverted)
Offers fast and sensitive 3D imaging, utilizing WF imaging, confocal imaging with 2 different pinhole sizes (40µm for higher contrast/25 µm for higher resolution) or TIRF (total internal reflection) imaging
-
- Excitation wavelengths (nm): 405-445-488-514-561-633
Emission filters: 450-525-600-647
*dichroic mirror deflects between GFP-RFP/CFP-YFP detection - Objectives:
- 5x/0.15 air
- 10X/0.1 air
- 25X/0.95 w
- 40X/1.1 w
- 63X/1.3 G
- 63X/1.4 O
- 100X/1.47 TIRF
- Multi-color fast acquisition as well as simultaneous dual-color imaging for all the above modes
- Finite-burst experiment design allows 2D dual-color simultaneous imaging at 100 fps (2048*2048 format)
- Enables fast, accurate navigation within large areas (16*14mm)
- 2 Zyla 4.2 camera with flexible AOI (area of interest) definition, binning (for enhanced sensitivity), and zoom options between 1X-1.5X-2X
- Mosaic for simultaneous, accurate illumination (photobleaching/photoactivation) of multiple areas on the sample
- Environmental control and auto-focus for live experiments, stage holder fitted to 35mm dishes, multi-well plates, and slides
- Excitation wavelengths (nm): 405-445-488-514-561-633
i. Fusion software offers GPU-enhanced deconvolution and instant Imaris view

Leica SP8-STED (Inverted)
Offers resolution up to 30nm lateral and ~100nm axial in 3D. combination of gated, spectral detection, and white light laser provides an excellent signal with full freedom in the choice of fluorophores at the visible range
- Lasers: 405, Ar (458, 476, 488, 496 and 514 nm), WLL (adjustable excitation 470-670 nm), STED dep lasers (594,660,775)
- Objectives:
- 10x/0.4 air
- 20x/0.75 air
- 40x/1.1w
- 63X/1.2w
- 63/1.4o
- 86x/1.2 w (STED)
- 93X/1.3 glycerol (STED)
- 100X/1.4 o (STED)
- 3HyD Detectors , 2PMTs
- Scanners- galvo/resonant
- Falcon -Lifetime imaging
Nano Live (Inverterd)
Nano live 3D cell explorer is a holo-tomographic microscope that measures the quantitative refractive index of cell organelles.
The system is equipped with an environmental control unit that controls the temperature and CO2 content inside the imaging chamber and enables the visualization of label-free unstained 3D cells.

|
Resolution |
Δx,y = 200nm; Δz = 400nm |
| Microscope Objective | Air with 60x magnification |
| Field of View | 85 x 85 x 30 μm |
| Tomography frame rate | Tomography frame rate |
Data for example:
Label-free Live Cell Imaging: Activated T-Cell Killing Cancer Cell
Label-free live cell imaging of Mesenchymal Stem Cells undergoing mitosis

Multiplexed imaging system composed from Akoya fluidics instrument that is connected to PhenoImager Fusion microscope.
The multiplexing capability of PhenoCycler-Fusion technology is based on PhenoCycler barcoding technology, each PhenoCycler antibody is conjugated to a unique oligonucleotide sequence that is complementary to a unique PhenoCycler reporter.
The PhenoCycler system can imagine a panel of ~40 antibodies on the same tissue section in one experiment.
Antibodies for Human FFPE/ FF or Mouse FF samples can be bought from PhenoCycler Akoya or manually conjugated to PhenoCycler barcodes.
The system provides single-cell resolution an imaging area of 15X34.6 mm2, and is equipped with a scientific CMOS camera.
Cytostretcher
The system of the Cytostretcher-LV can be installed on top of an inverted Leica DMI8 Wide-Field and equipped with an environmental control unit that controls the temperature and CO2 content inside the imaging chamber.
· The Cytostretcher-LV chambers are 5 mm x 5 mm single-well chambers (0.5 ml total volume).
· The Cytostretcher-LV is controlled by the Curi NaOMI software, which allows the user to design a stretch protocol and run the instrument.

Wet Lab
Wet Lab is available next to the microscopy rooms, for general sample preparation/processing/mounting.
The lab is equipped with
- General bench equipment
- Biological Hood and Incubator
- Basic Tissue Culture supply
- 4º, -20º refrigerators
- Chemical Hood
- Basic chemical lab equipment

Active clearing
- Tissue Clearing
we provide CLARITY based protocol suitable for different samples, reagents, suitable imaging platforms, and guidance during experimental set-up-
- Active Clearing: SmartClear II- Lifecanvas
- Based on stochastic electrotransport (Kim et al, PNAS, 2015) providing shorter clearing times without tissue damage or fluorescence quenching
- Pre-prepared clearing buffers, non-toxic, allow controlled and comparable process
- All clearing parameters (time, temperature, voltage, cycle shift) can be controlled and adjusted
- Various holder configurations for optimal clearing penetration for different sample sizes and composition
- Passive clearing: EasyClear
- Allows up to 10 independent samples (50ml tube size) to be cleared at different temperatures (range: 30-90 degC)
- Gentle shaking to maintain the morphology of delicate samples, with adjustable shaking angle and speed
- Index matching solution- EasyIndex
Suitable for clarity-based protocols and others, matches the refractive index of the sample to RI=1.46, allowing good fluorescence preservation
- Active Clearing: SmartClear II- Lifecanvas
-
Commercial software licenses
- arivis Vision4D (arivis) – Specialized visualization and analysis software for large 4D image datasets. We offer 1 floating license
-
arivis VisionVR – software that displays real image data in Virtual Reality (VR).
- Imaris (Bitplane/Oxford Instruments) – 4D analysis and visualization. We provide several floating licenses Imaris Stitcher
- AutoQuant (Media Cybernetics) – Deconvolotion software
- Huygens (SVI) - Deconvolution and analysis software
- LASx (Leica) - Acquisition, visualization and analysis software for data acquired on the Leica SP8 microscope
- Zen Blue (Zeiss) - Acquisition, visualization and analysis software for data acquired on the Zeiss microscopes (CD7)
- Zen Black (Zeiss) - Acquisition, visualization and analysis software for data acquired on the Z1 Lightsheet microscope.
- Analyze (Analyze Direct) – Visualization and Analysis software for medical imaging (MRI, uCT)
- Avizo & Amira (FEI) – 3D Visualization and Analysis software - several floating licenses are provided by the CRS department
- Volocity (Perkin Elmer) – floating license for visualization and analysis modules of version 6.1.1
Booking is made through Internal Services => Advanced Light Microscopy.
Workstations
Powerful Image Analysis Workstations (WS) are available for users of the MICC Cell Observatory in a dedicated classroom inside the facility (Ullmann basement room 28).
They can also be accessed remotely.
- 2 IA workstations are available at Ullman #28. Spec: Intel Xeon CPU E5-2620 @ 2.0 GHz, 64 GB of memory, NVIDIA Quadro 4000 graphic card. Booking is made through Internal Services => Bioimaging Queue
- UK WS 1,2: Spec: 2*Intel Xeon Gold 6130 CPU@2.1 GHz, 256 GB of memory, Nvidia Quadro P5000, 16GB graphic card, dedicated fast SSD disk for program cache.
- 3 very high-performance IA Workstations are dedicated to the processing of Lightsheet and other very big datasets at Ullman #28.
- Analysis 30T: 2*Intel Xeon E5-2690 CPU @ 2.6 GHz, 256GB of memory, Nvidia GeForce GTX TITAN X, 12GB graphic card
- Analysis 80T: 2*Intel Xeon E5-2690 CPU @ 2.6 GHz, 256GB of memory, Nvidia Quadro P5000, 16GB graphic card graphic card
- Analysis 20T, 20T2: 2*Intel Xeon E5-2667/2687 CPU@ 3.2 GHz, 512GB of memory, Nvidia Quadro M6000 24 GB graphic card, dedicated fast SSD disk for program cache
-
Wacom pen computer.
Booking is made through Internal Services => Advanced Light Microscopy.
All the above commercial software are available on the IA workstations, as well as: Fiji, Matlab, Python, and selected open-source software (e.g., Ilastik, QuPath, ICY, CellProfiler). Other open-source software can be installed on demand.
Data storage
In order to enable imaging data long-term storage and availability throughout the campus, the cell observatory maintains two types of data servers -
BioImg Server (700 TB) – Central long-term Storage Server for raw imaging data. Automatically collect imaging data from multiple campus-wide imaging platforms. Users can document, search, access, and share data from anywhere on campus. (BioImg step by step guide)
ESS server (500 TB) - A specially defined large storage with fast (10 Gbit) communication for data retention, analysis, and archiving, Supports the large data sets produced by light-sheet microscopes
