Our unit offers Weizmann Institute scientists access to advanced methods and expertise for the preservation, generation, and molecular engineering of antibodies developed in-house.
Each project begins with a personal consultation to define research goals and technical requirements. Together with the research team, we design a customized project plan that includes clearly defined milestones, timelines, and deliverables. Throughout the process, the unit provides scientific guidance, progress updates, and data-driven decision support to ensure the successful development of high-quality, validated antibody reagents tailored to each investigator’s specific needs.

Current Services
B cells from immunized animals are selected by Fluorescence-Activated Cell Sorting (FACS) based on their binding to specific antigens. They are then sequenced to identify the corresponding antibody gene that contains the genetic information for both the heavy and light chains of antibodies. The antibody can then be synthesized and expressed in cells to produce a recombinant monoclonal antibody for therapeutic or diagnostic use. The method combines high-throughput screening with traditional techniques to accelerate the generation of high-affinity antibodies. It enables the characterization of rare antibody-producing cells, the discovery of novel antibodies with desired specificities, and the understanding of B cell responses in health and disease.

Engineering the constant region of an antibody while keeping its antigen-binding regions unchanged. This process allows conversion between isotypes (e.g., IgG, IgA, IgM) to modify effector functions, stability, or tissue distribution. It enables researchers to optimize antibodies for therapeutic use without altering their antigen specificity.

Cloning and engineering the variable regions of an antibody into expression vectors containing constant regions from another species. This process preserves antigen specificity while adapting the antibody for compatibility with different experimental systems.

Engineering non-human antibodies to resemble human germline sequences while retaining antigen-binding specificity. By aligning variable regions with human germline frameworks and applying computational design principles, researchers can optimize both human compatibility and antigen affinity. This approach reduces immunogenicity and enhances therapeutic potential through structure-guided affinity improvement.

Engineered molecules that can bind two different antigens or epitopes simultaneously—often one on an immune effector cell and another on a target cell, such as a tumor. This dual specificity enables unique therapeutic mechanisms, such as redirecting immune cells to tumors, blocking two signaling pathways at once, or enhancing selectivity and efficacy compared to traditional monoclonal antibodies.

Engineering the high specificity of an antibody with the functional activity of another protein, such as a cytokine, enzyme, or receptor ligand. These fusion constructs can extend half-life, improve tissue targeting, or deliver bioactive payloads directly to diseased cells, offering powerful tools for targeted therapy or immune modulation.

Engineered Fabs and single-chain variable fragments (scFvs), represent smaller yet highly functional antibody derivatives. scFvs are composed of the VH and VL regions linked by a short peptide, retaining antigen-binding capacity in a compact form ideal for gene therapy, CAR-T design, and diagnostic imaging. These engineered and customized formats greatly expand antibody versatility, enabling advanced therapeutic, diagnostic, and research applications across diverse biomedical fields.

Existing hybridomas producing valuable monoclonal antibodies can lose productivity or change isotype over time. To preserve these important properties, the hybridoma is sequenced to archive their antibody genes immediately after their generation or upon receiving new hybridoma. In parallel, we perform hybridoma screening to confirm single-clone origin and ensure monoclonality, preventing mixed cultures that could compromise antibody quality, reproducibility, and long-term reliability.

Using adapted bioreactor systems to culture hybridoma or recombinant cell lines under controlled conditions. This approach enables high-yield, consistent production of antibodies with defined quality and purity, supporting research, diagnostic, and therapeutic applications.
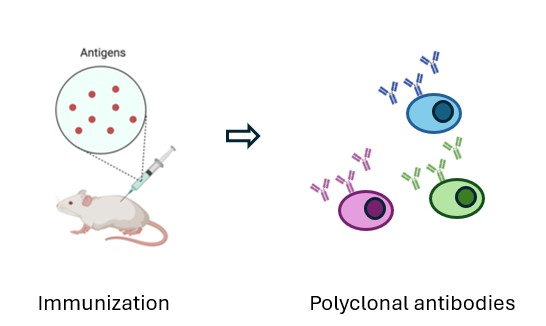
Polyclonal antibodies are generated by immunizing rats or mice with a target antigen, leading to the production of multiple antibody clones that recognize different epitopes. The resulting serum provides a broad and sensitive immune response, ideal for detection, screening, and validation applications.
Monoclonal antibodies against antigens are produced via fusing a mouse's antibody-producing B-cell with an immortal myeloma (cancerous) cell line, enabling it to establish cell-lines stably producing a single, highly specific monoclonal antibody (mAb) indefinitely. Selection and screening are carried out interactively with the users. Although labor intensive the method creates immortal cell lines that ensure a continuous, consistent antibody supply without repeated immunizations and remain a proven, reliable method with decades of successful use in research and therapeutics.

